Status
Completed
Title
Unintended effects of novel oral anticoagulants (NOAC) vs warfarin in real world settings vs warfarin
What were the objectives of the study?
Anticoagulants (also known as ‘blood thinning’ agents) are used to treat blood clots & also to help prevent people with irregular heartbeats from having a stroke. A stroke can be very serious as it can lead to loss of use of arms or legs, for example. Warfarin is a medicine used to treat blood clots and prevent strokes but it can cause problems for some patients. For example, it needs ongoing blood tests and can cause bleeding - such as vomiting or coughing up blood – which may need hospital care or could even be fatal.
There are some new anticoagulants which may not need blood tests but they are quite expensive. We are also not sure yet how safe these new treatments are in the longer term since the original trials were done over relatively short periods of time (only up to 2 years). Also the trials were done in selected patients who may be different from patients in real world settings. So we need to do research to find out how safe the new anticoagulants are compared with the older ones when used in real world settings over longer periods of time.
We also need to check whether patients who need the treatments are getting them (in other words, that no particular groups of patient are being overlooked). This can sometimes happen when new treatments are introduced (e.g. sometimes people from well off areas may get treatments when those from poorer areas don’t) so it’s important that we keep a look out for this.
How was the research done?
In the UK, we have a very large medical research database (QResearch) which has anonymised information on patients prescribed these drugs by their GP. This information is linked to data from hospitals and mortality records which means we can undertake our research to find out how safe these newer drugs are and whether they are being used appropriately. The results will be published so that everyone can benefit.
Chief Investigator
Julia Hippisley-Cox
Lead Applicant Organisation Name
Sponsor
Nottingham
Location of research
Nottingham
Date on which research approved
01-Jan-2016
Project reference ID
Q96
Generic ethics approval reference
03/04/021
Are all data accessed are in anonymised form?
Yes
Brief summary of the dataset to be released (including any sensitive data)
cohort of men and women prescribed anticoagulants between 2011 and 2016. Outcomes were major bleeding, death, cardiovascular disease or thrombosis on GP, hospital or mortality records.For full details see protocol
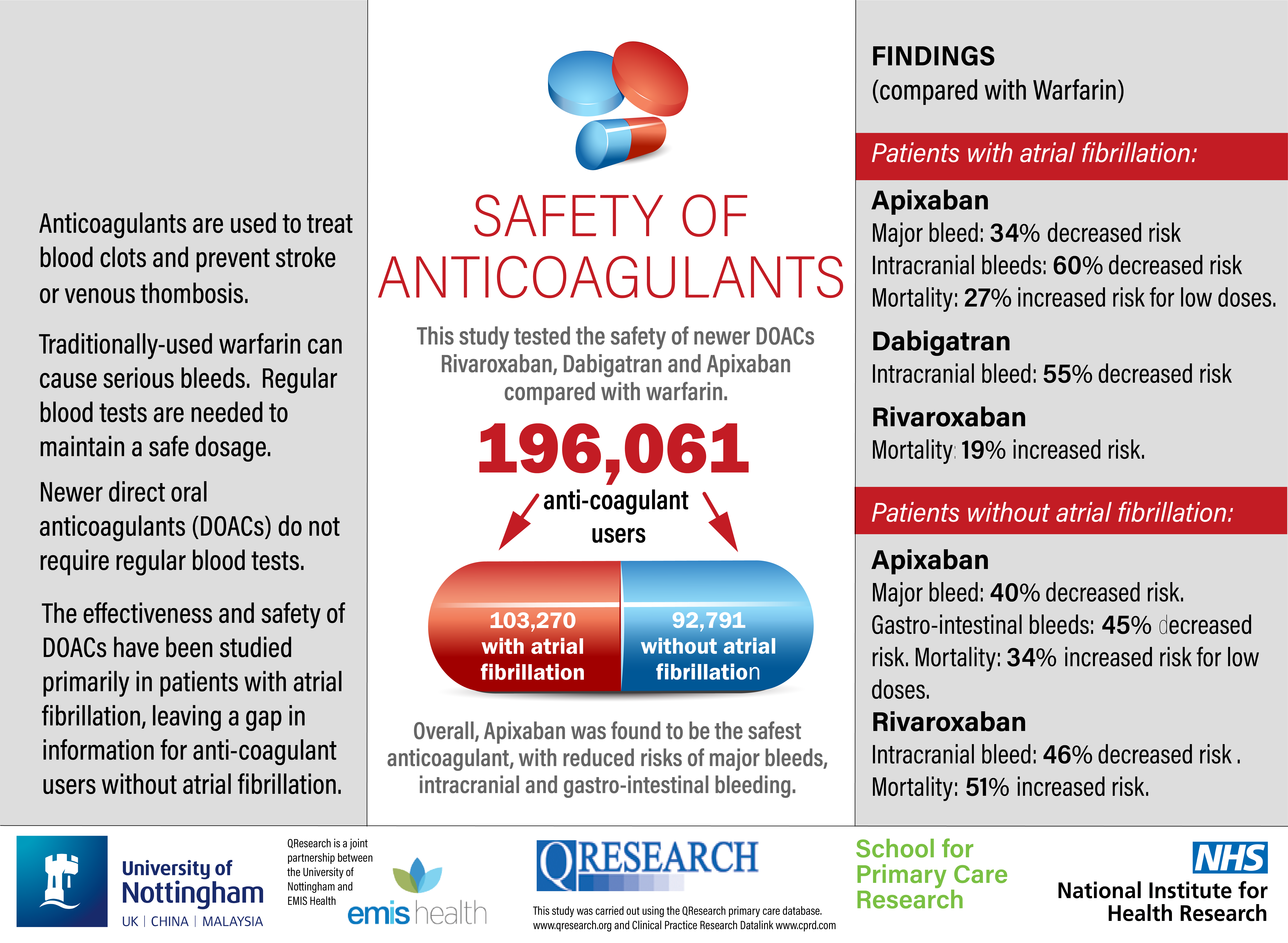
What were the main findings?
Comparing to warfarin, apixaban was associated with a decreased risk of major bleed in AF and non-AF patients. Rivaroxaban was associated with increased risk of all-cause mortality in both AF and non-AF patients. Weaknesses of the study include: uncertainty in adherence to medications; inconsistent recording of INR for warfarin users; and limited information about precise indication for prescribing.
Implications and Impact
Our study confirms previous findings for AF patients and extends them to non-AF patients. Increased risk of all-cause mortality in rivaroxaban users requires further research.
Funding Source
NIHR School for Primay Care Research
Public Benefit Statement
Research Team
Julia Hippisley-Cox, Carol Coupland, Yana Vinogradova: University of Nottingham
Publications
-
Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care
Authors: Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J
Ref: BMJ 2018; 362:k2505
https://www.bmj.com/content/362/bmj.k2505.long
Press Releases
- Real life GP data powers major anticoagulants study (21 June 2018)
Access Type
Trusted Research Environment (TRE)

