Status
Completed
Title
COVID-19 vaccination in children in England: uptake, safety and effectiveness
What were the objectives of the study?
We aim to investigate the uptake, safety and effectiveness of COVID-19 vaccines in children, in particular
1. To determine the uptake of COVID-19 vaccine administered to children in the age range of eligibility. Uptake of vaccines in children will be estimated in subgroups of sex, ethnicity, deprivation, geographic region of England, co-morbidities, prior COVID-19 status as well as by co-administration of other relevant childhood vaccinations.
2. To determine the safety profile of COVID-19 vaccine. We will a. evaluate the risk of each adverse event following each vaccination by dose and type; b. estimate how the risks of these adverse outcomes associated with vaccination compare with the risks associated with SARS-CoV-2 infection.
3. To evaluate the effectiveness of COVID-19 vaccine in children aged 5-15 years
COVID-19 vaccines: Astrazeneca (ChAdOx1), Pfizer (BNT162b2) and Moderna (mRNA-1273).
Eligibility of COVID-19 vaccination: 5-15 years (first, second and booster)
Chief Investigator
Dr Martina Patone
Lead Applicant Organisation Name
Sponsor
University of Oxford
Location of research
Oxford
Date on which research approved
07-Oct-2022
Project reference ID
OX167
Generic ethics approval reference
18/EM/0400
Are all data accessed are in anonymised form?
Yes
Brief summary of the dataset to be released (including any sensitive data)
Clinical outcomes which might indicate serious adverse events +
comorbidities + demographics and relevant information of the patients + influenza vaccine (using SNOMED codes)
Demo:
Sex
GP practice ID
Region of England
Townsend quintile
Ethnicity
Year of birth
Date left study population
Reason left study population
Date entered study population
BMI
Co-morbidities:
Chronic heart disease (congenital and acquired) (CHD)
Chronic respiratory disease (asthma, Cystic fibrosis, ciliary dyskinesia, bronchopulmonary dysplasia)
Gastrointestinal diseases
Asplenia or dysfunction of the spleen
Chronic conditions of the kidney, liver or digestive system
Diabetes diagnosis (any type 1 and type 2 diabetes)
Chronic neurological disease (including neurodisability, neuromuscular disease, degenerative neurological conditions)
Learning Difficulties (excluding down’s)
Neurodevelopment (autism and ADHD)
Mental Health (includes anxiety, depression and psychosis)
Cystic fibrosis
Epilepsy
Drugs (confounders):
4.2.1 Antipsychotic Drugs
4.3.1 Tricyclic And Related Antidepressant Drugs
4.3.2 Monoamine-Oxidase Inhibitors
4.3.3 Compound Antidepressant Preparations
4.3.3 Other Antidepressant Drugs
4.4 Central Nervous Stimulants
4.8.1 Drugs Used In Control Of Epilepsy
6.1.1 Insulins
6.1.1.1 Short-Acting Insulin Preparations
6.1.1.2 Intermediate And Long Acting Insulin Preparations
6.1.2 Oral Hypoglycaemic Drugs
Glinide or GPL-1 agonist or acarbose or guar etc
Suspected adverse events:
Febrile seizures
Guillain-Barre syndrome
Appendicitis
Chronic inflammatory
Syncope
Demyelinating
Neuropathy
Injection site reaction
Myositis
Pancreatitis
Seizure
Myocarditis
Anaphylaxis
Angioedema
Thrombocytopenia
Rash
Enlarged lymph nodes
Headache
Nausea and vomiting
Fever
Clinical outcomes which might indicate serious adverse events + comorbidities (using ICD-10 codes)
Co-morbidities:
Chronic heart disease (congenital and acquired) (CHD)
Chronic respiratory disease (asthma, Cystic fibrosis, ciliary dyskinesia, bronchopulmonary dysplasia)
Gastrointestinal diseases
Chronic conditions of the kidney, liver or digestive system
Diabetes diagnosis (any type 1 and type 2 diabetes)
Rare genetic, metabolic and autoimmune diseases
Transplant
Chronic neurological disease (including neurodisability, neuromuscular disease, degenerative neurological conditions)
Learning Difficulties (excluding down’s)
Suspected adverse events:
Guillain-Barre syndrome
Appendicitis
Chronic inflammatory
Demyelinating
Neuropathy
Myositis
Pancreatitis
Seizure
Myocarditis
Anaphylaxis
Angioedema
Thrombocytopenia
Multi system inflammatory syndrome
Date of death, cause of death including where the cause of death indicates one of the clinical outcomes which might indicate serious adverse events (using ICD-10 codes)
Suspected adverse events:
Guillain-Barre syndrome
Appendicitis
Chronic inflammatory
Demyelinating
Neuropathy
Myositis
Pancreatitis
Seizure
Myocarditis
Anaphylaxis
Angioedema
Thrombocytopenia
Multi system inflammatory syndrome
We will incl. NIMS (COVID-19 vaccination data) and SGSS/Pillar2 (SARS-CoV-2 testing data)
Funding Source
NIHR School for Primary Care Research
Public Benefit Statement
Research Team
Professor Julia Hippisley-Cox (University of Oxford)
Professor Carol Coupland (University of Nottingham)
Dr Defne Saatci (University of Oxford)
Dr Sharon Dixon (University of Oxford)
Dr Jennifer Hirst (University of Oxford)
Miss Emma Coupland (University of Oxford)
Approval Letter
Publications
-
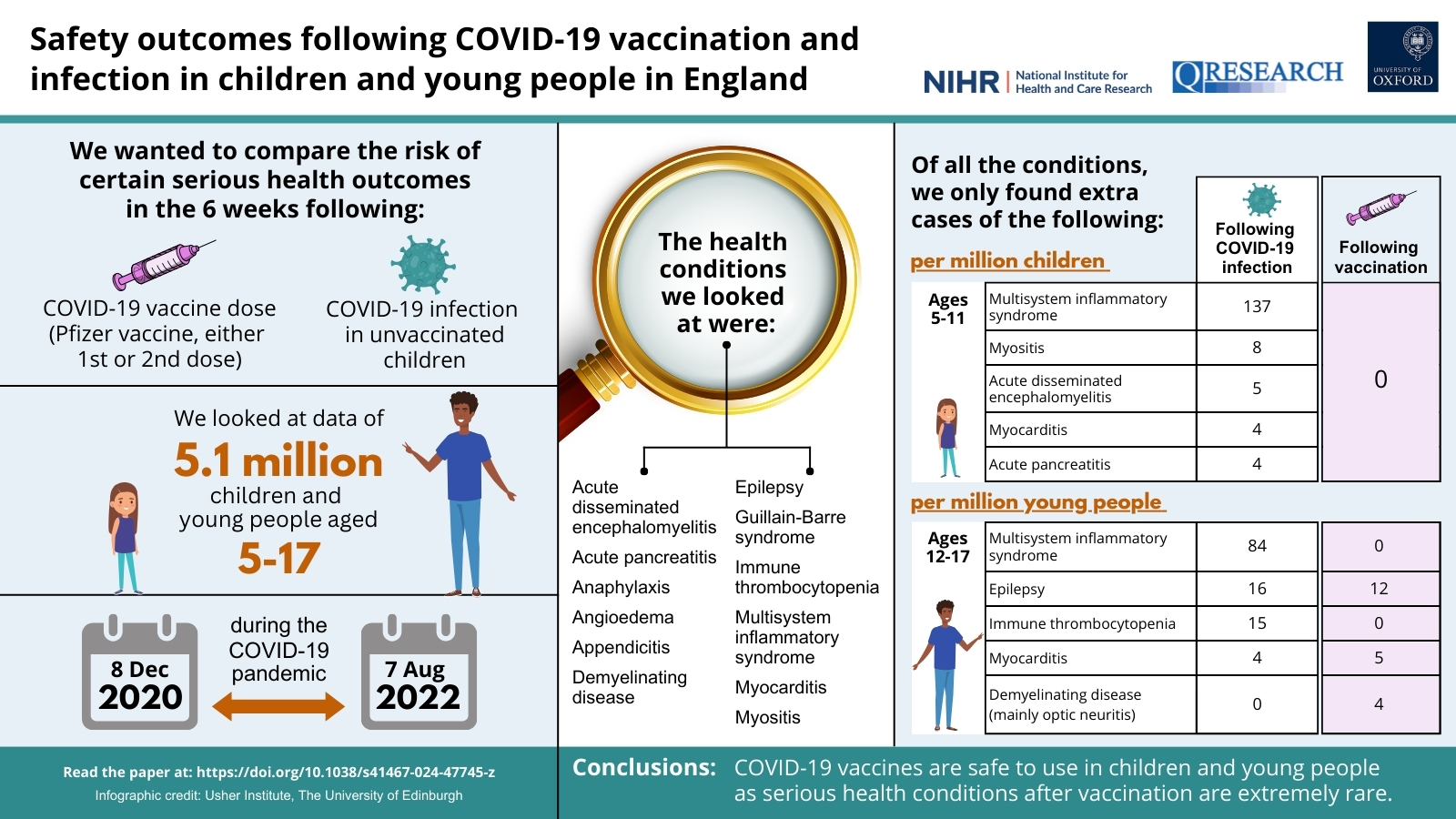
Safety outcomes following COVID-19 vaccination and infection in 5.1 million children in England
Authors: Emma Copland, Martina Patone, Defne Saatci, Lahiru Handunnetthi, Jennifer Hirst, David P. J. Hunt, Nicholas L. Mills, Paul Moss, Aziz Sheikh, Carol A. C. Coupland, Anthony Harnden, Chris Robertson, Julia Hippisley-Cox
Ref:
https://link.springer.com/article/10.1038/s41467-024-47745-z?utm_source=rct_congratemailt&utm_medium=email&utm_campaign=oa_20240527&utm_content=10.1038%2Fs41467-024-47745-z
Press Releases
Access Type
Trusted Research Environment (TRE)

