Status
Completed
Title
Uptake and comparative safety of new COVID-19 therapeutics by age, sex, region, ethnicity, comorbidities, medication, deprivation, risk level and evidence of prior COVID infection
What were the objectives of the study?
COVID-19 has affected millions of people around the world and has had major consequences for peoples’ health, work and lives. This made it important to quickly develop vaccines to help protect people against COVID-19.
Several vaccines and other medicines to help prevent or treat COVID-19 have been approved for use in the UK and are now being given in the general population. All these were shown to be safe in the trials that tested them before they were approved. It is also really important to see how these treatments work when they start being used in the real-world. This is because real-world populations can be different from the populations included in vaccine trials, for example, people in trials might be younger or have less health problems. Monitoring treatments in the real-world includes looking at how safe the vaccine is, and how well it works in the wider world, for example in older patients, people with various illnesses and those from different ethnic backgrounds.
As well as safety, another important thing to monitor is whether there are patterns that show differences in who is having each treatment (i.e. uptake). It is crucial to monitor uptake, because if there are population groups who are not having the treatments, then this could create or worsen inequalities in health. Knowing about these patterns means interventions can be designed that could help increase vaccine uptake to improve this.
One important way that the new treatments can be monitored is by using the information in healthcare records about what happens when the treatment is given. This includes looking at whether the treatment is working and whether there are symptoms recorded after that treatment has been given that might suggest unexpected side effects.
General practice (GP) patient records contain the information needed to answer these questions quickly and on very large numbers of people. The QResearch database contains millions of anonymised GP records which can be used by researchers to answer these questions.
This project will assess uptake of the COVID-19 vaccination programme and monitor the safety of the new COVID-19 treatments using the QResearch database.
Chief Investigator
Professor Julia Hippisley-Cox
Lead Applicant Organisation Name
Sponsor
Oxford
Location of research
University of Oxford
Date on which research approved
19-Jan-2021
Project reference ID
OX107
Generic ethics approval reference
18/EM/0400
Are all data accessed are in anonymised form?
Yes
Brief summary of the dataset to be released (including any sensitive data)
GP data on demographics, risk factors for COVID-19, co-morbidities,
Hospital data including clinical outcomes which might indicate serious adverse events
Mortality data including date of death, cause of death including where the cause of death indicates one of the clinical outcomes which might indicate serious adverse events (see indicator list in protocol)
Cancer registry dates and diagnoses of cancer, chemotherapy, radiotherapy
ICNARC data, transplant data and COVID-19 test results.
ONS occupation data
National Immunisation Database
Monoclonal antibody and antiviral datasets
Funding Source
Health Data Research UK (HDR-UK); Oxford NIR BRC; NIHR Health Technology Program
Public Benefit Statement
Research Team
The researchers are from the Universities of Oxford, Cambridge, Nottingham, Leicester, Guys and Thomas's, NHS Blood and Transplant Service, Kings College London, the Intensive Care National Audit and Research Centr (ICNARC).
Julia Hippisley-Cox, Carol Coupland, Peter Watkinson, Kathy Rowan, David Harrison, Fergus Gleeson, Manu Shankar-Hari, Anthony Harnden, Douglas Thornburn, Tom Ranger, Winnie Mei, Pui San Tan, Martina Patone, Rommel Ravanan, Kamlesh Khunti, Francesco Zaccardi, Simon Griffin, Chris Callaghan
Approval Letter
Publications
-
Uptake, effectiveness, and comparative safety of new COVID-19 vaccines by age, sex, region, ethnicity, comorbidities, medication, deprivation, risk level and evidence of prior COVID infection
Authors: Hippisley-Cox J, Patone M, May W, Tan PS, Watkinson P, Rowan K, Harrison H, Shankar-Hari M, Thorburn D, Khunti K, Zaccardi F, Griffin S, Callaghan C, Coupland CA.
Ref:
https://www.qresearch.org/media/1304/ox107_covid_vaccine_safety_protocol.pdf -
Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study
Authors: Julia Hippisley-Cox, Martina Patone, Xue W Mei, Defne Saatci, Sharon Dixon, Kamlesh Khunti, Francesco Zaccardi, Peter Watkinson, Manu Shankar-Hari, James Doidge, David A Harrison, Simon J Griffin, Aziz Sheikh, Carol A C Coupland
Ref:
https://www.bmj.com/content/374/bmj.n1931 -
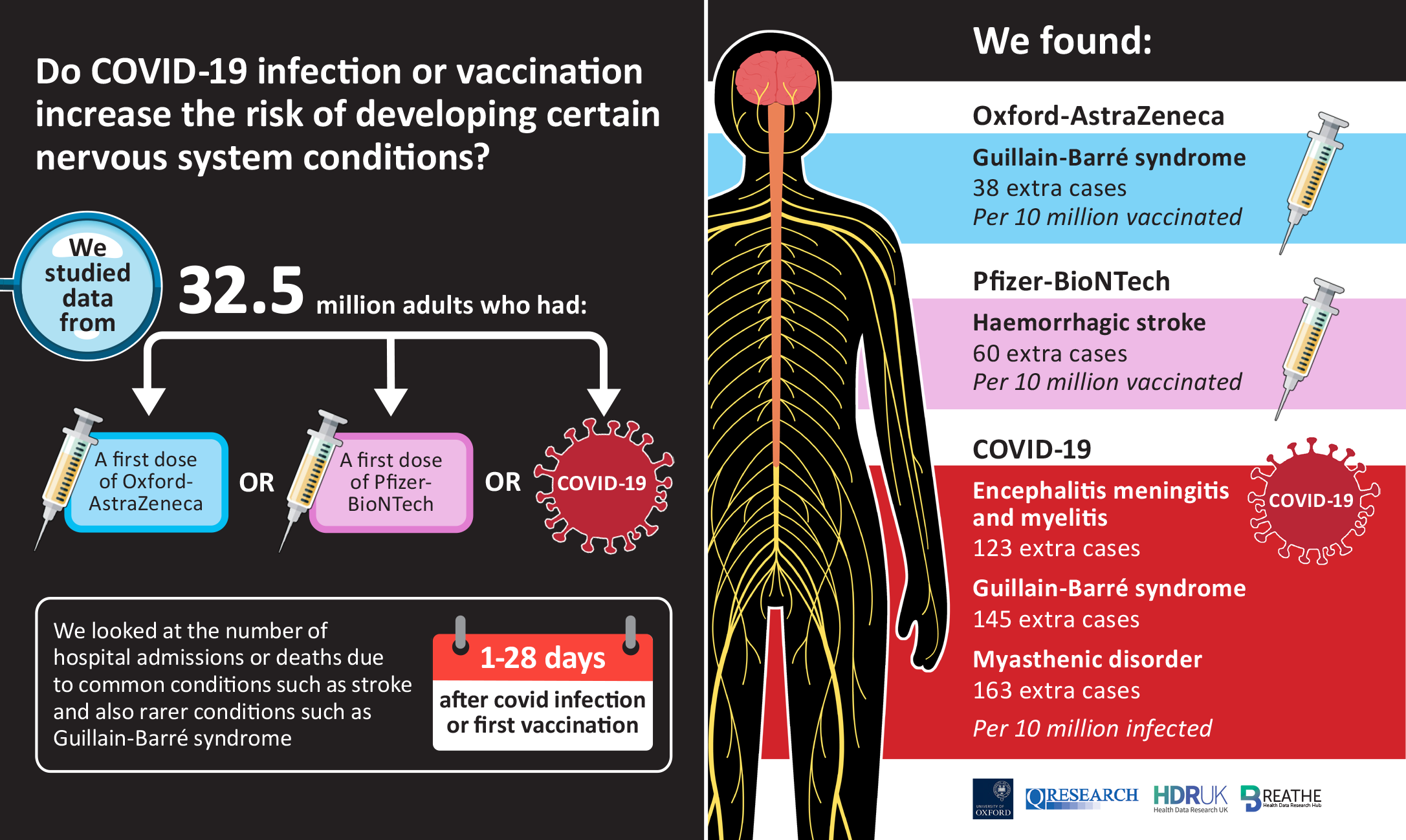
Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection
Authors: Martina Patone, Lahiru Handunnetthi, Defne Saatci, Jiafeng Pan, Srinivasa Vittal Katikireddi, Saif Razvi, David Hunt, Xue W. Mei, Sharon Dixon, Francesco Zaccardi, Kamlesh Khunti, Peter Watkinson, Carol A. C. Coupland, James Doidge, David A. Harrison, Rommel Ravanan, Aziz Sheik, Chris Robertson, Julia Hippisley-Cox
Ref:
https://www.nature.com/articles/s41591-021-01556-7.epdf?sharing_token=2EEiP94twaMC-qRxtlpFk9RgN0jAjWel9jnR3ZoTv0M_gNB_ZF4A8MyPfNNqp7LM5P_p2NkItT-Y5x2WPrJPNg4cFrjQq4xy3X8geR6P-Z7enVo7o2uGjuJuuDuLG6dUbzuN0z9iVZYeGdLYPJ5pBhG57HjlTy2OUDK0CmHJfOw%3D -
Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection
Authors: Martina Patone, Xue W. Mei, Lahiru Handunnetthi, Sharon Dixon, Francesco Zaccardi, Manu Shankar-Hari, Peter Watkinson, Kamlesh Khunti, Anthony Harnden, Carol A. C. Coupland, Keith M. Channon, Nicholas L. Mills, Aziz Sheikh, Julia Hippisley-Cox
Ref:
https://www.qresearch.org/media/1360/s41591-021-01630-0-1-1.pdf -
Risk of myocarditis following sequential COVID-19 vaccinations by age and sex
Authors: Martina Patone, Xue W Mei, Lahiru Handunnetthi, Sharon Dixon, Francesco Zaccardi, Manu Shankar-Hari, Peter Watkinson, Kamlesh Khunti, Anthony Harnden, Carol AC Coupland, Keith M. Channon, Nicholas L Mills, Aziz Sheikh, Julia Hippisley-Cox
Ref:
https://www.medrxiv.org/content/10.1101/2021.12.23.21268276v1.article-metrics -
Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: a population-based cohort study
Authors: Carmen Piernas, Martina Patone, Nerys M Astbury, Min Gao, Aziz Sheikh, Kamlesh Khunti, Manu Shankar-Hari, Sharon Dixon, Carol Coupland, Paul Aveyard, Julia Hippisley-Cox, Susan A Jebb
Ref:
https://www.thelancet.com/action/showPdf?pii=S2213-8587%2822%2900158-9 -
Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex
Authors: Martina Patone, Xue W. Mei, Lahiru Handunnetthi, Sharon Dixon, Francesco Zaccardi, Manu Shankar-Hari, Peter Watkinson, Kamlesh Khunti, Anthony Harnden, Carol A.C. Coupland, Keith M. Channon, Nicholas L. Mills, Aziz Sheikh and Julia Hippisley-Cox
Ref:
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.122.059970 -
QCovid 4 - Predicting risk of death or hospitalisation from COVID-19 in adults testing positive for SARS-CoV-2 infection during the Omicron wave in England
Authors: Julia Hippisley-Cox, Kamlesh Khunti, Aziz Sheikh, Jonathan S Nguyen-Van-Tam, Carol AC Coupland
Ref:
https://www.medrxiv.org/content/10.1101/2022.08.13.22278733v1 -
Impact of vaccination on COVID-19-associated admissions to critical care in England: a population cohort study of linked data
Authors: David A Harrison, Peter J Watkinson, James C Doidge, Manu Shankar-Hari, Paul R Mouncey, Martina Patone, Carol A C Coupland, Julia Hippisley-Cox, Kathryn M Rowan
Ref:
https://www.medrxiv.org/content/10.1101/2022.10.03.22280649v1 -
Uptake of Sotrovimab for prevention of severe COVID-19 and its safety in the community in England
Authors: Martina Patone, Andrew JHL Snelling, Holly Tibble, Carol AC Coupland, Aziz Sheikh, Julia Hippisley-Cox
Ref:
https://www.medrxiv.org/content/10.1101/2022.08.17.22278893v1 -
Uptake, effectiveness and safety of COVID-19 vaccines in the immunocompromised population: A population-based cohort study in England
Authors: Chen D, Copland E, Hirst J, Mi E, Dixon S, Coupland CAC, Hippisley-Cox J
Ref:
https://www.researchsquare.com/article/rs-3316645/v1 -
Uptake, efectiveness and safety of COVID-19 vaccines in individuals at clinical risk due to immunosuppressive drug therapy or transplantation procedures: a population-based cohort study in England
Authors: Daniel Tzu‑Hsuan Chen1, Emma Copland, Jennifer A. Hirst, Emma Mi, Sharon Dixon, Carol Coupland, Julia Hippisley‑Cox
Ref:
https://link.springer.com/content/pdf/10.1186/s12916-024-03457-1.pdf
Press Releases
- Health Data Research UK selects 12 projects to accelerate use of data for vital COVID-19 research
- Covid-19, not vaccination, presents biggest blood clot risks 27th August 2021
- COVID-19 infection has greater risk of causing very rare neurological events than vaccines
- AstraZeneca and Pfizer vaccines associated with rare but potentially serious side effects in study
- Risk of debilitating nerve syndrome almost four times higher from Covid than vaccine
- Covid-19 more likely to cause neurological issues than vaccines, study finds
- Covid: Vaccine study links virus to rare neurological illness
- ‘Covid-19 presents greater risk of rare neurological events than vaccines’
- Covid linked to higher risk of neurological complications than vaccines, study finds
- AstraZeneca vaccine and Covid both linked to increased risk of Guillain-Barré syndrome
- Greater risk for neurological complications from COVID-19 infection vs. vaccination, analysis shows
- What you need to know about COVID-19 vaccines and rare neurological complications
- Covid vaccines associated with rare neurological complications, says study
- New study shows risk of heart inflammation following COVID-19 infection and vaccination
- AstraZeneca: A Vaccine for the World?
- COVID-19 vaccine protects people of all body weights from hospitalisation and death, study of 9 million adults in England suggests
- Myocarditis Risk Low Overall After COVID-19 Vax, but Higher in Some Groups
- Study shows disparity in COVID-19 vaccines uptake for immunocompromised individuals, despite evidence of vaccine effectiveness and safety
Access Type
Trusted Research Environment (TRE)

