Status
Completed
Title
Optimising NHS blood cancer patient care in the COVID-19 pandemic
What were the objectives of the study?
Covid has affected millions of people around the world and has had a major impact on their health, work and lives. This made it important to quickly develop vaccines to help protect people against Covid. Several vaccines have been approved for use in the UK and continue to be given to the general population. Before these vaccines were approved, they were tested in trials and deemed to be safe.
As the pandemic continues alongside a Covid vaccination programme, it is becoming increasingly more important to monitor how these vaccines work when given to patients with blood cancer. This is because blood cancer patients may have a different immune response to those included in the trials.
Blood cancer patients may have weakened immune systems, which could make them more prone to severe Covid should they become infected with SARS-CoV-2. Severe Covid is when someone has more serious side effects when contracting the virus, such as becoming seriously ill and being hospitalised, or dying.
This is because some forms of cancers mean that those affected may have weakened immune systems, and therefore might not be able to generate a ‘good response’ to the vaccine and be less protected against the virus. The effectiveness of these vaccines may also vary among people who are receiving different treatments for other types of blood cancers, as each of these treatments may have different effects on the immune system.
Covid vaccines are generally very safe and effective, but some small studies have suggested that people with blood cancers may have a poorer vaccine response, potentially leaving them less protected. This might especially be the case for patients receiving a type of therapy called anti-CD20 therapy, or stem cell transplants.
However, stronger, larger scale evidence is needed to provide clarity to people living with blood cancers and their clinical teams on how safe and effective these vaccines are for them, and how to safely care for them as the pandemic continues. It may be that people with some types of blood cancer – or those who receive specific treatments – need booster doses or may respond better to one specific vaccine. This study will use a large healthcare database that has collected data throughout the pandemic to examine the risks of severe Covid in people with blood conditions – such as their risk of hospitalisation, intensive care admission or death – compared to people who did not have blood cancer. It will then look into the uptake of vaccines in people with these conditions and their sub-types and assess whether the risk of severe side effects varies among people with different blood disorders. The data for this study will be anonymised, and will only be accessed by a small team of researchers on secure servers at the University of Oxford. The database collects routine medical data from general practices, and registered patients can opt out of this.
Lastly, it will investigate how effective each of the three currently used vaccines in the UK are in protecting people with blood cancer. They will do this by comparing specific groups, such as sub-types of cancers, and different treatments patients may currently be on.
How was the research done?
This study will use several research methods depending on the aims being addressed.
The first method will be a ‘cohort’ study, which will use the data collected from 24th January 2020 (date of the first confirmed SARS-CoV-2 infection in the UK) throughout both waves of the pandemic. This approach will be used to assess the risks of individuals with blood cancers in terms of being hospitalised, admitted to intensive care, or dying from Covid. The risks of Covid will be evaluated among different sub-groups, such as the types of blood cancer (e.g., leukaemia), or types of treatment (e.g., chemotherapy).
The second will involve a method called ‘Kaplan-Meier methodology’, which will measure the uptake of each vaccine type in people with blood cancers/non-cancer blood disorders over time.
The third will involve a method called a ‘self-controlled case series’ to assess if the risks of severe side effects associated with vaccinations (such as blood clots) are different in people with blood disorders.
The fourth will use a matched cohort study – people that received a vaccine will be matched with a person that did not receive a vaccine. They will then compare these respective groups based on their risk of getting infected with SARS-CoV-2 and becoming seriously ill or dying from Covid.
Once the vaccine efficacy results have been calculated for everyone in the study (overall effectiveness), they will then evaluate the results within the following groups: blood cancers overall, non-cancerous blood disorders overall (such as sickle cell), sub-groups of blood cancers, and by different treatment groups (e.g., those that have received a bone marrow transplant).
Chief Investigator
Prof Julia Hippisley-Cox
Lead Applicant Organisation Name
Sponsor
University of Oxford
Location of research
University of Oxford
Date on which research approved
04-Oct-2021
Project reference ID
OX300
Generic ethics approval reference
18/EM/0400
Are all data accessed are in anonymised form?
Yes
Brief summary of the dataset to be released (including any sensitive data)
GP data on age, demographics (ethnicity, region and deprivation), diagnoses of blood cancers, sickle cell, and other conditions that confer risk to severe Covid (e.g., diabetes), prescriptions of medications including immune suppressants, and records of bone marrow/other transplants.
Chemotherapy data regarding type of medication used for blood cancers.
Hospital data on diagnoses, treatments and status of blood cancers and non-cancerous blood disorders.
Implications and Impact
The Covid pandemic in the UK continues to fluctuate and although a vaccination campaign has been rolled out, there is substantial concern among people with blood cancers, other blood disorders and their care teams, as to how much protection they actually have after being vaccinated.
Having a deeper understanding on the risks of severe Covid in this very diverse group of conditions will complement evidence from larger studies on vaccine safety and effectiveness for these patients. Results from this study may identify that some individuals could need ‘booster’ vaccine doses, that some vaccines may be more effective and should be a preferred option for different blood disorders, as well as being used to understand the ongoing risks from Covid in these people.
Current evidence is limited, as a very small number of people were involved in these studies. Therefore, using a large database may provide far more robust results and answers for people affected by blood cancer.
Funding Source
Blood Cancer UK (https://bloodcancer.org.uk/)
Public Benefit Statement
Research Team
Prof Julia Hippisley-Cox, University of Oxford
Prof Carol Coupland, University of Oxford
Prof Mark Middleton, University of Oxford
Dr Chris Cunningham, Oxford University Hospitals NHS Trust
Dr Ashley K. Clift, University of Oxford
Dr Martina Patone, University of Oxford
Publications
-
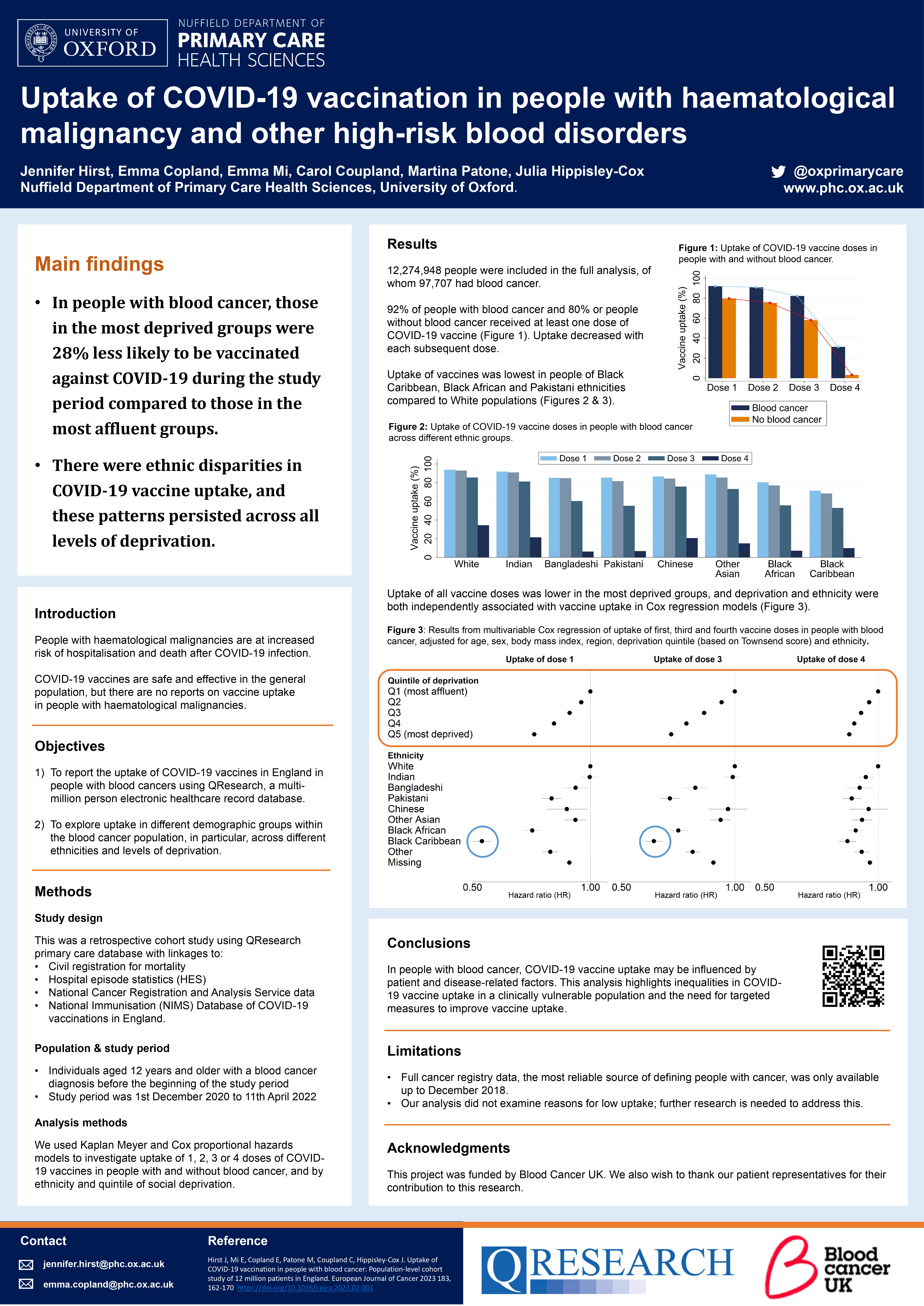
Uptake of COVID-19 vaccination in people with blood cancer: Population-level cohort study of 12 million patients in England
Authors: Hirst J, Mi E, Copland E, Patone P, Coupland C, Hippisley-Cox J
Ref:
https://www.ejcancer.com/article/S0959-8049(23)00059-X/fulltext -
Effectiveness and safety of COVID-19 vaccination in people with blood cancer
Authors: Emma Copland, Jennifer Hirst, Emma Mi, Martina Patone, Daniel Chen, Carol Coupland, Julia Hippisley-Cox
Ref:
https://www.ejcancer.com/article/S0959-8049(24)00079-0/fulltext
Press Releases
Access Type
Trusted Research Environment (TRE)
